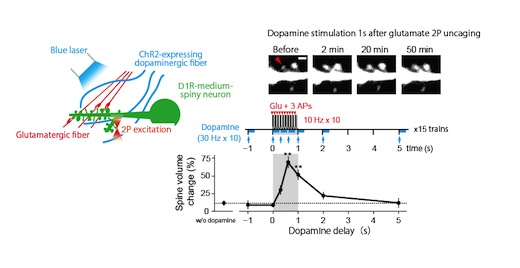
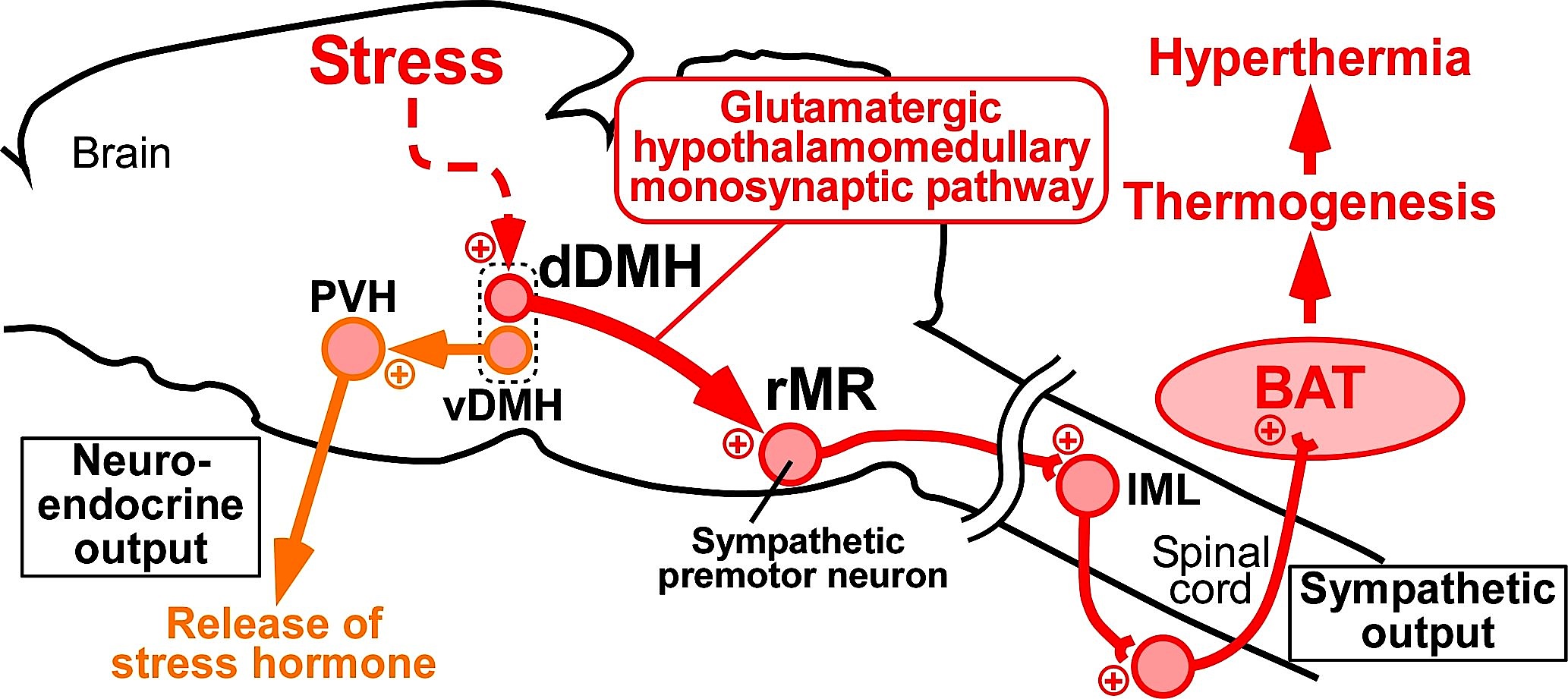
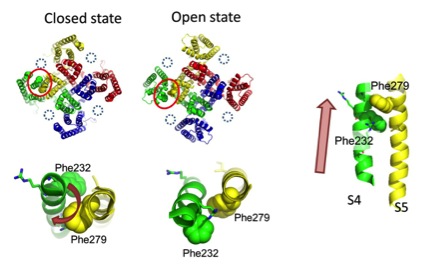
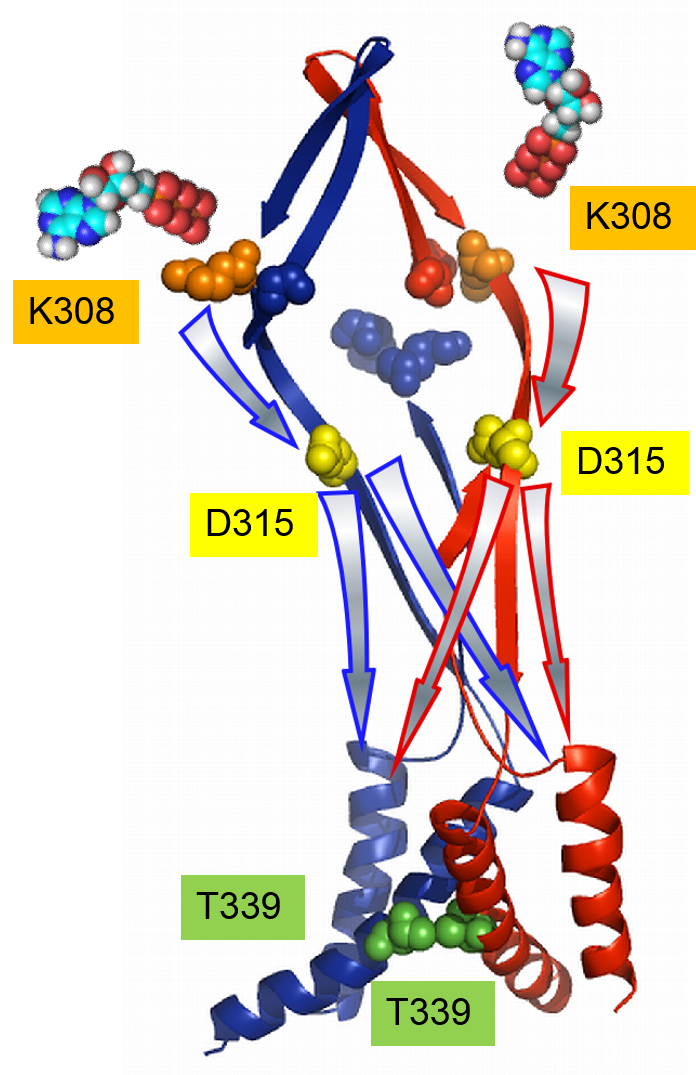
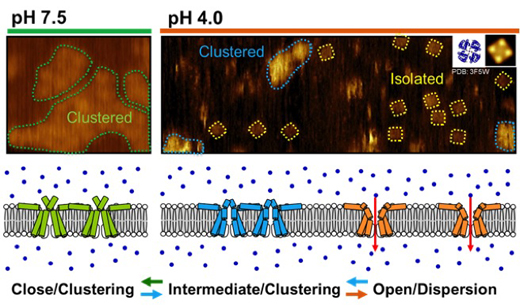
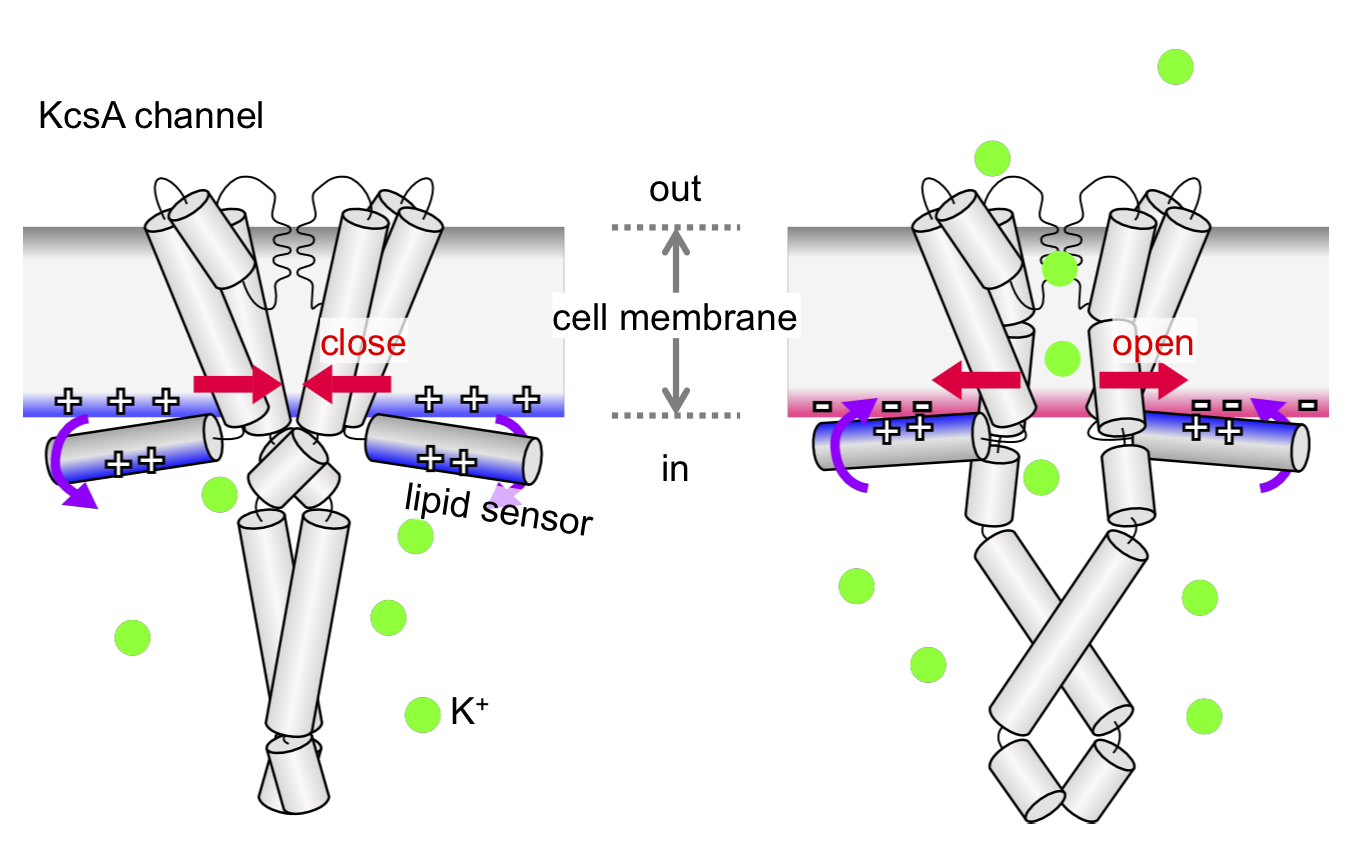
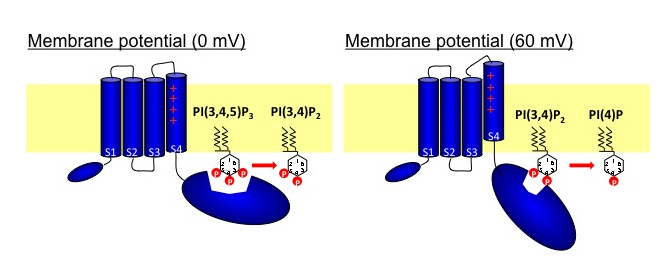
Neuronal computation circuits are represented by brain structures. Ocular dominance columns (ODCs) have been well studied in the striate cortex (V1) of macaques, as well defined arrays of columnar structure. When ODCs were first identified in 1960’s, researchers thought that this structure represents circuit of stereoscopic depth coding. However, ODC expression seems obscure in some New World primate species, although they are all capable of stereopsis. Then, studies of depth coding through ODCs have been turned down. Previously, ODCs have been investigated by means of eye injections of transneuronal transporters and examination of cytochrome oxidase (CO) activity patterns after monocular enucleation. Here, we used the expression of immediate-early genes (IEGs), c-Fos and Zif268, after monocular inactivation (MI) to identify ODCs in New World owl monkeys. Using IEGs, we not only revealed apparent ODCs in owl monkeys but also discovered a number of unique features of their ODCs. These ODCs sometimes bridged to other columns in layer 4. The ODC pattern continued into V2. Finally, border strips were observed along ODC borders after only brief MI. Our data suggest that apparent species differences of ODCs were due to technical limitations, not fundamental nature, and revive debate over the functions, variability and development of ODCs.
Takahata et al., Identification of ocular dominance domains in New World owl monkeys by immediate-early gene expression. Proc Natl Acad Sci USA, (2014) 111 (11): 4297-4302
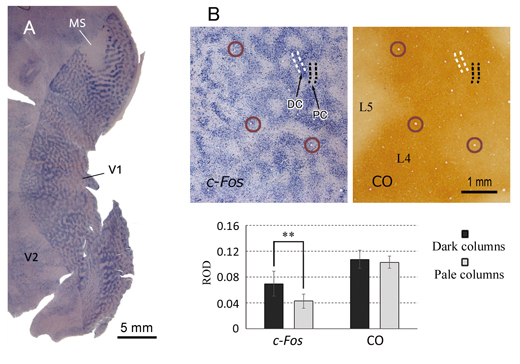
Figure. A: In situ hybridization for c-Fos in a tangential section of flattened V1 of an owl monkey that was subjected for MI. Apparent ODCs were represented by the staining pattern.B: ODCs are not revealed by cytochrome oxidase (CO) staining that was commonly used to reveal ODCs. Adjacent sections were stained for c-Fos or CO. While c-Fos staining shows significant difference in relative optical density (ROD) between pale and dark columns, CO staining does not. Circles represent identical radial blood vessels of adjacent sections.
Department of Psychology, Vanderbilt University, USA
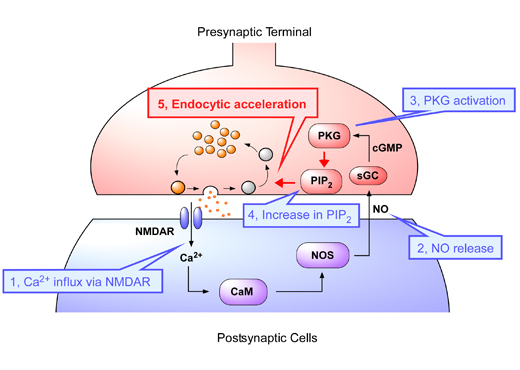
We are able to detect slight changes in the musical rhythm. Recent studies suggest that the cerebellum and basal ganglia are involved in temporal processing for non-motor cognitive functions (e.g. rhythm perception and temporal judgment; Fig. B). Here we show that neurons in the cerebellar dentate nucleus encode the interstimulus interval of isochronous rhythm and play a crucial role in predicting the timing of the next stimulus.
We trained monkeys to detect a single omission of isochronous repetitive stimuli (Fig. A). We found that the dentate neurons responded to each stimulus and gradually elevated the response as the repetition progressed, opposed to the sensory adaptation. The magnitude of the response positively correlated with the interstimulus interval (Fig. C). Because inactivation of the recording sites delayed the detection of stimulus omission, these signals might be necessary for the prediction of stimulus timing. This study revealed the underlying mechanisms of rhythm perception at single-neuron level. Our findings will advance the understanding of the cerebellar disorder and encourage future clinical techniques for the diagnosis and the evaluation of treatment.
Ohmae S, Uematsu A, Tanaka M. “Temporally specific sensory signals for the detection of stimulus omission in the primate deep cerebellar nuclei.” J Neurosci 2013; Sep 25
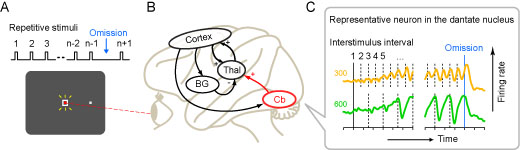
(A) Missing oddball task. During monkeys looked at the fixation point (red dot) on the monitor, visual stimuli (white square) were presented repeatedly at a fixed interval. Tones were also presented simultaneously. As monkeys reported the omission of the audiovisual stimulus by making a saccadic eye movement, drops of juice were given as a reward. (B) Neural circuitry related to the temporal processing. Cb, Cerebellum; Thal, Thalamus; BG, Basal ganglia; Cortex, Cerebral cortex. (C) Example of a neuron recorded from the cerebellar dentate nucleus. In the left and right panels, data were aligned with the first stimulus and the omission of the stimulus, respectively. Vertical dashed line indicates the timing of each repetitive stimulus. Note that the response was greater as the repetition progressed and the interstimulus interval was longer.
1Department of Physiology, Hokkaido University School of Medicine (2Department of Psychology, University of Pennsylvania)
]]>