Science Topics - 79
Voltage-sensing phosphatase (VSP) consists of the two domains: voltage-sensor domain and the cytoplasmic region with phosphoinositide-phosphatase activities. The phosphatase region exhibits remarkable sequence similarity to PTEN, a tumor suppressor phosphatase. VSPs dephosphorylate the 5′ position of the inositol ring of both phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] and phosphatidylinositol 4,5-bisphosphate [PI (4,5)P2] upon voltage depolarization. However, it is unclear whether VSPs also have 3′ phosphatase activity. To gain insights into this question, we performed in vitro assays of phosphatase activities with radiolabeled PI(3,4,5)P3. TLC assay showed that the 3′ phosphate of PI(3,4,5)P3 was not dephosphorylated, whereas that of phosphatidylinositol 3,4-bisphosphate [PI(3,4)P2] was removed by VSPs. Monitoring of PI(3,4)P2 levels with the pleckstrin homology (PH) domain from tandem PH domain containing protein (TAPP1) fused with GFP (PHTAPP1-GFP) by confocal microscopy in amphibian oocytes showed an increase of fluorescence intensity during depolarization to 0 mV, consistent with 5′ phosphatase activity of VSP toward PI(3,4,5)P3. However, depolarization to 60 mV showed a transient increase of GFP fluorescence followed by a decrease, indicating that, after PI(3,4,5)P3 is dephosphorylated at the 5′ position, PI(3,4)P2 is then dephosphorylated at the 3′ position. These results suggest that substrate specificity of the VSP changes with membrane potential.
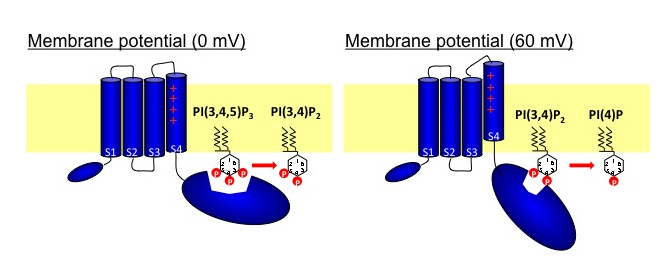
Kurokawa T, Takasuga S, Sakata S, Yamaguchi S, Horie S, Homma KJ, Sasaki T, Okamura Y, PNAS, 109: 10089-10094 (2012)
