Science Topics - 93
P2X2 receptor channel, a homotrimer activated by binding of extracellular ATP to three intersubunit ATP binding sites (each located ~50Å from the ion permeation pore), also shows voltage-dependent activation upon hyperpolarization. Here, we used tandem trimeric constructs (TTC) harboring critical mutations at the ATP-binding, linker, and pore regions to investigate how ATP-activation signal is transmitted within the trimer and how signals generated by ATP and hyperpolarization converge. Analysis of voltage-and [ATP]-dependent gating in these TTCs showed that: (1) Voltage- and [ATP]-dependent gating of P2X2 requires binding of at least two ATP molecules. (2) D315A mutation in the beta-14 strand of the linker region connecting the ATP binding domains to the pore-forming helices induces two different gating modes; this requires presence of D315A mutation in at least two subunits. (3) T339S mutation in the pore domains of all three subunits abolishes the voltage dependence of P2X2 gating in saturating [ATP], making P2X2 equally active at all membrane potentials. Increasing the number of T339S mutations in the TTC results in gradual changes in the voltage-dependence of gating from that of the wild-type channel, suggesting equal and independent contributions of the subunits at the pore level. (4) Voltage- and [ATP]-dependent gating in TTCs differs depending on the location of one D315A relative to one K308A that blocks the ATP binding and downstream signal transmission. (5) Voltage- and [ATP]-dependent gating does not depend on where one T339S is located relative to K308A (or D315A). Our results suggest that each intersubunit ATP binding signal is directly transmitted on the same subunit to the level of D315 via the domain that contributes K308 to the beta-14 strand. The signal subsequently spreads equally to all three subunits at the level of the pore, resulting in symmetric and independent contributions of the three subunits to pore opening.
Batu Keceli and Yoshihiro Kubo, Signal transmission within the P2X2 trimeric receptor. Journal of General Physiology (2014) 143: 761-782 (Published online May 26 2014, 10.1085/jgp.201411166)
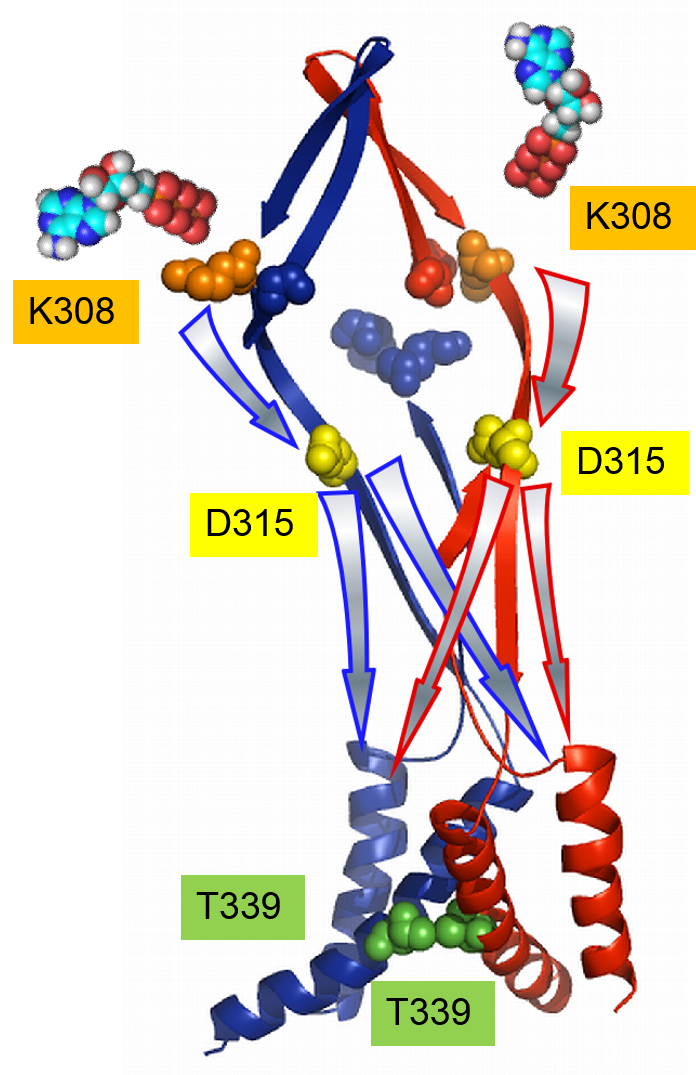
Figure. Schematic presentation of the activation-signal transmission from two ATP binding sites to the pore upon voltage- and [ATP]-dependent activation.
For simplicity, two subunits are illustrated in red and blue. Colored spheres mark the K308 (orange) and K69 (blue) residues in the ATP binding region, D315 (yellow) in the linker, and T339 (green) at the pore level. Arrows with the same color of borderlines with the subunits depict the signal transmission on each subunit. The activation signal from one inter subunit ATP binding flows directly on the corresponding beta-14 strand of the ATP-binding site down to the D315 level, and then spreads to other subunits at the pore level.
Division of Biophysics and Neurobiology, Department of Molecular Physiology, National Institute for Physiological Sciences
