Science Topics - 95
In human heart, the slowly-activating delayed rectifier potassium current, also known as IKs, plays a key role in regulating cardiac excitability. KCNQ1 and its auxiliary subunit KCNE1 underlie the IKs current by forming an ion channel complex. Although KCNQ1 itself can produce potassium current, co-expression of KCNQ1 and KCNE1 produces slowly-activating and deactivating potassium current with a large positive shift (+40 mV) of the G-V curve. In other words, KCNE1 makes KCNQ1 channel much harder to be activated, yet the molecular mechanism has been largely unknown.
In this work, we found that Phe232 on the S4 segment (the center of voltage sensor) of KCNQ1 and Phe279 on the S5 segment from the neighbor KCNQ1 subunit are in proximity and collide during the upward movement of the voltage sensor during activation of the channel in the presence of KCNE1. We also applied voltage clamp fluorometry to record the voltage sensor movement and the current simultaneously. We found that the transition from the intermediate state (in which the voltage sensors are in the up state yet the pore is closed) to the open state is delayed due to the steric hindrance between the two phenylalanine residues (Phe232 and Phe279).
These results indicate that the interaction between the voltage sensor domain and the pore domain of KCNQ1 is modified by the presence of KCNE1, and makes the channel harder to be activated. Steric hindrance of the bulky phenylalanine residues plays a main role in creating the collision between the two domains.
Nakajo, K. & Kubo, Y. Steric hindrance between S4 and S5 of the KCNQ1/KCNE1 channel hampers pore opening. Nature Communications 5:4100, doi: 10.1038/ncomms5100 (2014).
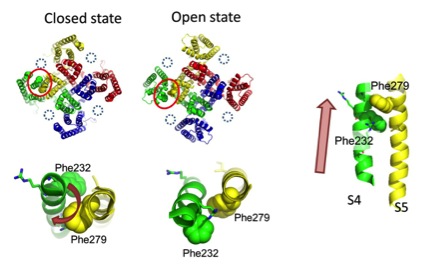
Figure. Two phenylalanine residues collide during activation of KCNQ1/KCNE1 channel
