Science Topics - 81
Calcium-dependent protein phosphatase calcineurin (CaN) is a key molecule to govern pathological cardiac hypertrophy. CaN dephosphorylates a downstream transcription factor NFAT, which in turn up-regulates hypertrophy-related genes. However, little is known about how CaN is activated independently of excitation-contraction coupling. Here, we found that the Na+/H+ exchanger NHE1 activates the CaN-NFAT signaling, leading to cardiomyocyte hypertrophy via direct binding of CaN to the 6-residues motif (PVITID) of NHE1. Over-expression of NHE1 promoted nuclear translocation and promoter activity of NFAT, which requires the clustering of NHE1 in lipid rafts. Importantly, increasing pH strongly activates CaN, thus we hypothesize that NHE1 produces a localized microdomain with higher pH, thereby sensitizing CaN to activation and promoting NFAT signaling. This is the first report showing unexpected functional coupling between pH-regulator NHE1 and Ca2+-dependent enzyme CaN.
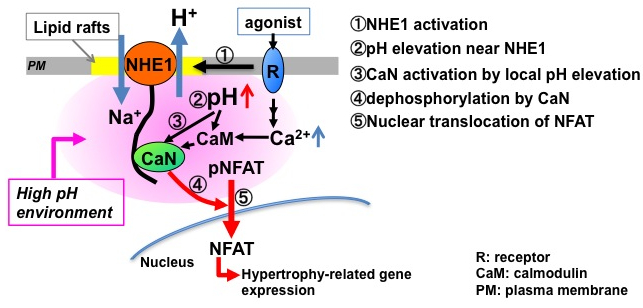
Figure: Upon stimulation of NHE1 with receptor agonists (1), localized microdomain with higher pH is formed (2). The pH elevation potentiates the activity of CaN bound to NHE1 via increased Ca2+ sensitivity to CaN (3), and then promotes dephosphorylation of NFAT (4). The dephosphorylated NFAT translocates into nucleus (5), leading to the hypertrophy-related gene expression. This mechanism highlights an important role of NHE1 as a spatial and transient platform for CaN activation.
Hisamitsu T, Nakamura TY and Wakabayashi S, Mol. Cell. Biol. 32:3265-3280, 2012
Department of Molecular Physiology, National Cerebral and Cardiovascular Center
