Science Topics - 92
The pH-dependent potassium channel, KcsA, has been extensively studied for the molecular mechanism of the gating. Crystallographic studies of the KcsA channel revealed the closed structure at neutral pH and the open structure at acidic pH. These crystallographic structures were obtained, however, for the channels extracted from the membrane (the detergent-solubilized form), thus, the native structure of the channel in the membrane-embedded condition still remains unsolved. In this study, we have directly observed the structure of the KcsA channel in the membrane using atomic force microscopy (AFM). With high-resolution imaging, we have successfully resolved the closed-gate structure at neutral pH and the open-gate structure at acidic pH. In the membrane, the open-gate channels were sparsely dispersed without contacting each other. On the other hand, we found surprisingly that the channels were clustered at neutral pH. To examine the time course of the clustering–dispersion dynamics we applied high-speed AFM. Upon pH changes, the channels underwent clustering–dispersion dynamics within several minutes, and reversible clustering–dispersion occurred for repeated pH changes. At acidic pH, a small fraction of the channels remained clustered, in which the channels did not open but changed the conformation slightly from the closed one (the intermediate gating structure). These results suggest that upon acidic change the channel undergoes the conformational change slightly in the cluster, and then opens the gate once the channel is dispersed as singly-isolated channels. This unprecedented collective behavior of the KcsA channel, gating-coupled clustering–dispersion in the membrane, gives clues of the complicated behavior of channel proteins on the cell membrane.
(Sumino, A., Yamamoto, D., Iwamoto, M., Dewa, T., Oiki, S.: Gating-Associated Clustering–Dispersion Dynamics of the KcsA Potassium Channel in a Lipid Membrane. J. Phys. Chem. Lett. 5: 578–584, 2014; Sumino, A., Sumikama, T., Iwamoto, M., Dewa, T., and Oiki, S.: The Open Gate Structure of the Membrane-Embedded KcsA Potassium Channel Viewed From the Cytoplasmic Side. Scientific Reports 3: 1063, 2013.)
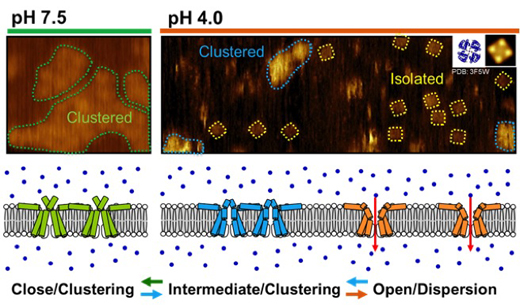
Figure. Gating-coupled clustering–dispersion of the pH-dependent potassium channel, KcsA.
At neutral pH, the closed channels are clustered in which each channel is resolved as a round shape (left). Upon acidic change, the channels take slightly shortened but closed conformation in the cluster (the intermediate conformational state; center), and gradually they are dispersed as singly-isolated channels (right). The KcsA channel is a tetrameric channel, and the isolated channel has square shape with the pore at the center (an averaged image; right inset).
1JST/PRESTO, 2Department of molecular physiology and biophysics, Faculty of medical sciences, University of Fukui
