Science Topics – 113
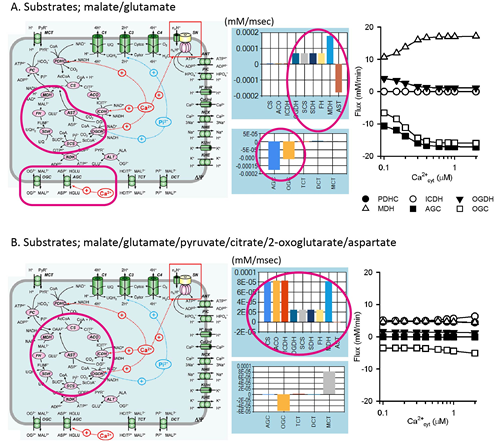
The heart is continuously pumping blood through the body by utilizing hydrolysis energy of a huge amount of ATP. The ATP is mainly produced by oxidative phosphorylation in mitochondria. It is well known that the energy metabolites such as ATP, ADP and NADH, are kept relatively constant during physiological cardiac workload transition. Although Ca2+ has been suggested to be one of the possible mechanisms, the details are unclarified yet. In the present study, we constructed a mathematical model of cardiac mitochondria based on experimental data and studied whether known Ca2+-dependent regulation mechanisms play roles in the metabolite constancy. Model simulations revealed that the Ca2+-dependent regulation mechanisms have important roles under the “in vitro” condition of isolated mitochondria where malate/glutamate are used as energy substrates. Under this condition, only a half of TCA cycle is active, and Ca2+-dependent regulation of malate-aspartate shuttle, especially aspartate/glutamate carrier AGC, contributes to the Ca2+-dependent metabolite constancy (Fig. A). On the other hand, under the “in vivo” condition where all substrates are used, whole components of a TCA cycle are active, and the contribution of a Ca2+-dependent regulation of malate-aspartate shuttle becomes smaller (Fig. B). Therefore, the contribution of Ca2+-dependent regulation mechanisms might be limited under the physiological condition.
Saito R, Takeuchi A, Himeno Y, Inagaki N, Matsuoka S. A simulation study on the constancy of cardiac energy metabolites during workload transition. The Journal of Physiology, 594(23): 6929-6945, 2016.
<Figure Legends>
Contribution of Ca2+-dependent regulation mechanisms to the constancy of cardiac energy metabolites. A. Under the malate/glutamate condition, only a half of a TCA cycle is active, and contribution of Ca2+-dependent activation of malate-aspartate shuttle is large. B. Under the full substrates condition, whole components of a TCA cycle are active, and the contribution of Ca2+-dependent activation of malate-aspartate shuttle becomes smaller.
1Biology Research Laboratories, Mitsubishi Tanabe Pharma Corporation, 2Department of Diabetes, Endocrinology and Nutrition, Graduate School of Medicine, Kyoto University, 3Department of Integrative and Systems Physiology, Faculty of Medical Sciences, University of Fukui, 4Department of Physiology and Biophysics, Graduate School of Medicine, Kyoto University
